Mastering organic chemistry requires diligent practice, specifically with nomenclature. This practice set, featuring 50 compounds for naming and drawing, builds a strong foundation.
What is Organic Chemistry Nomenclature?
Organic chemistry nomenclature is a systematic method for naming organic compounds, ensuring each unique structure has a distinct and unambiguous identifier. This isn’t simply memorization; it’s understanding a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
These rules dictate how to identify the parent chain, functional groups, and substituents, then combine these elements into a standardized name. Practice sets, like those containing 50 compounds for naming, are crucial for internalizing these principles. Documents detailing structural, displayed, and skeletal formulas, alongside requests for IUPAC names, exemplify this practice.
Understanding nomenclature is fundamental, as it allows chemists to communicate effectively and accurately about molecular structures, even without visual representations. The provided resources, including PDFs and practice exercises, aim to solidify this essential skill.
Importance of Naming Practice
Consistent practice in organic chemistry nomenclature is paramount for success. A solid grasp of naming conventions isn’t merely about recalling rules; it’s about developing a logical approach to structure identification and communication. Resources like practice sets with 50 compounds – both naming and drawing exercises – are invaluable tools.
These exercises reinforce the ability to decipher complex structures and translate them into systematic IUPAC names, and vice versa. Documents presenting compounds with varying representations (structural, displayed, skeletal) demand a comprehensive understanding.
Furthermore, proficiency in nomenclature is essential for interpreting scientific literature and accurately conveying information in research. Mastering this skill prevents ambiguity and fosters clear communication within the scientific community, building a strong foundation for advanced studies.

Basic Nomenclature Rules
Understanding IUPAC guidelines is crucial. Practice sets, like those with 20 examples, emphasize classifying, naming, and recognizing isomers – foundational skills for success.
IUPAC Nomenclature System
The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic approach to naming organic compounds, ensuring clarity and avoiding ambiguity. This system relies on a set of rules that define how to identify and name different functional groups and structural features within a molecule.

Effective practice, utilizing resources like naming and drawing exercises with 50 compounds, is essential for mastering these rules. These exercises often present structures requiring identification of the parent chain, functional groups, and substituents. Understanding displayed and skeletal formulas, as seen in practice documents, is also vital;
Consistent application of IUPAC rules allows chemists worldwide to communicate about specific compounds without confusion. Documents containing multiple-choice questions and compound examples reinforce this standardized language, building a strong foundation for more complex organic chemistry concepts.
Identifying the Parent Chain
Determining the parent chain is the foundational step in IUPAC nomenclature. This involves locating the longest continuous carbon chain within the molecule, representing the base structure for naming. Practice sets, containing structures of varying complexity, are crucial for developing this skill.
Often, identifying the parent chain isn’t straightforward, especially in branched or cyclic compounds. Documents with structural, displayed, and skeletal formulas challenge students to accurately discern the longest chain. This skill is reinforced through exercises requiring both naming compounds from structures and drawing structures from names.
Consistent practice with these 50-compound sets builds proficiency in recognizing the parent chain, even when multiple possibilities exist. Mastering this step is essential for correctly applying IUPAC rules and accurately naming organic molecules.
Functional Group Prioritization
Once the parent chain is established, prioritizing functional groups becomes critical. IUPAC nomenclature assigns a specific order of precedence to different functional groups – carboxylic acids take priority over ketones, for example. Practice sets, like those containing 50 compounds, force students to apply this hierarchy consistently.
These practice materials often present molecules with multiple functional groups, demanding accurate identification and correct prioritization. Documents showcasing structural formulas alongside naming exercises reinforce this concept. Understanding this order dictates which group defines the suffix and which are named as prefixes.
Successfully navigating complex nomenclature requires memorization of the priority list and consistent application through practice. The provided sets, focusing on both naming and drawing, solidify this understanding, preparing students for more advanced organic chemistry concepts.

Alkanes and Alkyl Groups
Initial practice focuses on simple alkanes, then progresses to branched structures. Nomenclature PDFs provide examples, building skills for naming and drawing these fundamental compounds.
Naming Simple Alkanes
Beginning with straightforward alkanes is crucial for grasping organic chemistry nomenclature. These compounds – methane, ethane, propane, butane, and pentane – form the basis for understanding more complex structures. Practice PDFs often dedicate significant sections to these foundational examples, providing structures for students to name and names for students to draw.
The core principle involves identifying the longest continuous carbon chain, which dictates the parent alkane name. For instance, a five-carbon chain is pentane. These initial exercises reinforce this concept, building confidence before tackling branched alkanes. Consistent practice with these simple structures, utilizing available online resources and practice sets, is paramount for success. Mastering these basics unlocks the ability to systematically name more intricate organic molecules.
Many introductory PDFs include quizzes specifically focused on simple alkane naming, offering immediate feedback and reinforcing learning.
Branched Alkane Nomenclature
Moving beyond straight-chain alkanes, branched alkanes introduce the concepts of alkyl groups and numbering. Organic chemistry practice PDFs emphasize identifying the longest carbon chain including branches, then numbering it to provide the lowest possible numbers for the substituents. These substituents are named as alkyl groups – methyl, ethyl, propyl, and so on – and their positions are indicated by the corresponding carbon numbers.
Practice sets frequently present structures with multiple branches, requiring students to systematically name each substituent and its location. PDFs often include examples demonstrating how to alphabetize substituents when multiple different groups are present. Mastering this requires consistent application of the IUPAC rules, and dedicated practice with naming and drawing branched alkanes is essential.
Effective PDFs will provide step-by-step solutions to complex examples, clarifying the naming process and building confidence.
Cycloalkanes: Naming and Structure
Cycloalkanes, cyclic hydrocarbons, require a slightly modified naming approach. Organic chemistry practice PDFs highlight the prefix “cyclo-” added to the alkane name corresponding to the number of carbon atoms in the ring. For example, a three-carbon ring is cyclopropane, a four-carbon ring is cyclobutane, and so on.
Substituents on cycloalkanes are named similarly to those on alkanes, but the ring itself is the parent chain. Numbering begins at a carbon bearing a substituent, aiming for the lowest possible numbers. PDFs often include structures with multiple substituents, demanding careful numbering and alphabetical ordering.
Practice sets also emphasize drawing accurate ring structures, understanding bond angles, and recognizing the inherent strain in smaller rings. Consistent practice with naming and sketching cycloalkanes is crucial for solidifying understanding.

Common Functional Groups and Their Nomenclature
Organic chemistry practice PDFs focus on alcohols, aldehydes, ketones, carboxylic acids, and esters. Mastering their IUPAC rules is vital for accurate compound identification.
Alcohols and Phenols: Naming Conventions
Practicing alcohol and phenol nomenclature is crucial, as these functional groups present specific naming challenges. Organic chemistry practice PDFs emphasize identifying the longest carbon chain containing the –OH group for alcohols.
Numbering begins to prioritize the hydroxyl group, assigning it the lowest possible number. Prefixes like ‘hydroxy’ are used, and the parent alkane name is modified accordingly. For phenols, the –OH group is directly attached to a benzene ring.
These are named as hydroxylated benzenes, often using common names alongside IUPAC names (e.g., phenol itself). Practice sets frequently include compounds with multiple hydroxyl groups or combined with other functional groups, demanding a thorough understanding of prioritization rules. Careful attention to detail is key for accurate naming.

Aldehydes and Ketones: IUPAC Rules
IUPAC nomenclature for aldehydes and ketones focuses on identifying the carbonyl group (C=O). Aldehydes, where the carbonyl is at the end of the chain, are named with the suffix “-al” added to the parent alkane, with carbon one being the carbonyl carbon – no numbering is usually needed.
Ketones, with the carbonyl group internal to the chain, utilize the suffix “-one”. Numbering prioritizes the carbonyl carbon, ensuring it receives the lowest possible number. Organic chemistry practice PDFs often present structures requiring careful chain identification.
Compounds with substituents necessitate including their positions in the name. Mastering these rules, through consistent practice with naming and drawing exercises, is vital for success. Pay close attention to prioritizing the carbonyl group during numbering.
Carboxylic Acids and Esters: Naming Practices
Carboxylic acids are named by replacing the terminal “-e” of the corresponding alkane with “-oic acid”. The carboxyl carbon (C=O-OH) is always carbon number one, eliminating the need for explicit numbering in simple structures. Practice PDFs will often include examples with multiple substituents.
Esters are derived from carboxylic acids and alcohols, named as alkyl alkanoates. The alkyl portion comes from the alcohol, and the alkanoate portion from the carboxylic acid. Identifying these components is key.
Complex esters may require careful attention to substituent positions. Consistent practice with naming and drawing, utilizing resources like organic chemistry practice sets, solidifies understanding. Remember to correctly identify the parent acid and alcohol components.

Complex Nomenclature Scenarios
Advanced practice PDFs present compounds with multiple functional groups, heterocycles like morpholine and triazole, and isomeric forms, demanding precise IUPAC naming skills.
Compounds with Multiple Functional Groups
Naming organic molecules boasting several functional groups requires a systematic approach prioritizing based on the IUPAC rules. Practice PDFs often feature compounds containing alcohols, ketones, carboxylic acids, and amines simultaneously. The highest priority functional group dictates the suffix, while others become prefixes.
For instance, a molecule with both a hydroxyl (-OH) and a carboxylic acid (-COOH) group necessitates identifying the carboxylic acid as the primary functional group, leading to a carboxylic acid name with “hydroxy” as a prefix. Mastering this prioritization is crucial.
These practice sets frequently include examples involving complex arrangements, challenging students to correctly identify and name each functional group in the correct order. Consistent practice with these scenarios solidifies understanding and minimizes errors in nomenclature.
Naming Compounds with Heterocycles (Morpholine, Triazole)
Heterocyclic compounds, like those containing morpholine (tetrahydro-1,4-oxazine) or 1,2,4-triazole rings, present unique naming challenges. Practice PDFs dedicated to organic chemistry nomenclature often include these structures to test understanding of specialized rules. Morpholine is typically named as a substituent, while triazole requires identifying the nitrogen positions.
When a heterocyclic ring is part of a larger molecule, it’s treated as a substituent, and its name is incorporated as a prefix. The numbering within the ring is crucial for accurate naming, especially with triazoles where positional isomers exist.
These practice exercises emphasize recognizing these ring systems and applying the correct IUPAC prefixes and suffixes. Successfully naming these compounds demonstrates a comprehensive grasp of organic nomenclature principles.
Isomers and Stereochemistry in Nomenclature
Organic chemistry naming practice PDFs frequently incorporate isomers to assess understanding beyond basic connectivity. Isomers, possessing the same molecular formula but different arrangements, require careful distinction through IUPAC nomenclature;
Stereochemistry – the 3D arrangement of atoms – adds another layer of complexity; cis/trans designations for alkenes and cyclic compounds, and R/S configurations for chiral centers, are vital components of accurate naming. Practice sets often present structures requiring these stereochemical descriptors.
Identifying constitutional isomers (different connectivity) and stereoisomers (different arrangement) is key. Mastering these concepts, reinforced through dedicated practice, is crucial for correctly naming and interpreting organic molecules.

Resources for Practice
Numerous online PDFs offer organic chemistry naming practice. Sets with 50 compounds – both naming and drawing exercises – are readily available for focused skill development.
Online Organic Chemistry Naming Practice PDFs
A wealth of online resources provides organic chemistry naming practice in PDF format, catering to diverse learning styles and skill levels. These PDFs frequently contain comprehensive sets of compounds, often exceeding 20 examples, designed to reinforce IUPAC nomenclature rules. Many focus on identifying parent chains, functional groups, and applying prioritization principles.
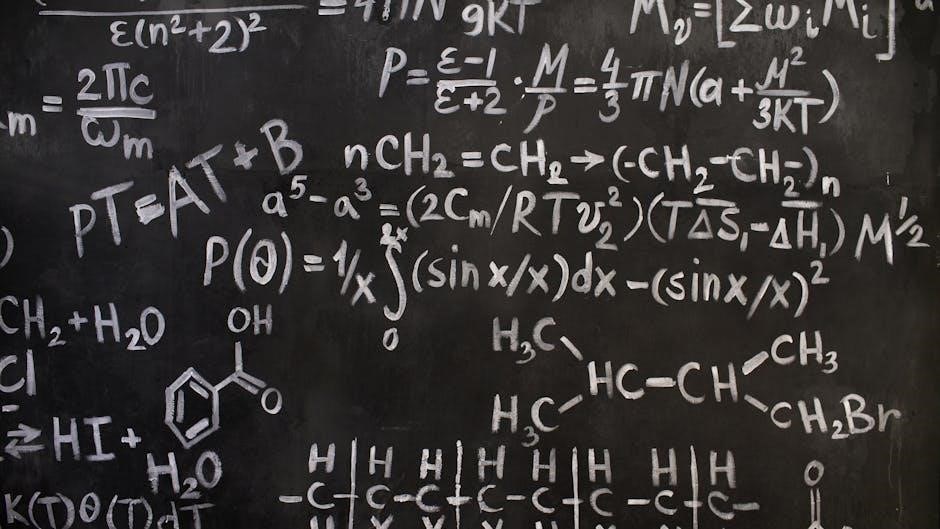
Specifically, documents featuring 50 compounds for both naming and drawing structures are commonly found. These resources often include multiple-choice questions, mirroring exam formats, and detailed answer keys for self-assessment. Some PDFs showcase structural, displayed, and skeletal formulas, enhancing visual understanding. Furthermore, resources addressing complex scenarios – compounds with multiple functional groups or heterocycles like morpholine and triazole – are available;

Students can leverage these PDFs for targeted practice, focusing on areas where they encounter difficulty. Consistent engagement with these materials is crucial for mastering organic chemistry nomenclature and building confidence.
Practice Sets with 50 Compounds (Naming & Drawing)
Dedicated practice sets containing 50 organic compounds – requiring both naming from structures and drawing structures from names – are invaluable for solidifying understanding. These sets often mirror the format of quizzes and exams, providing realistic assessment opportunities. The compounds span a range of functional groups, including alkanes, alcohols, aldehydes, ketones, carboxylic acids, and esters.
Effective practice involves systematically applying IUPAC nomenclature rules, identifying the parent chain, prioritizing functional groups, and correctly representing stereochemistry where applicable. Working through these sets helps students recognize common patterns and avoid frequent errors. Resources may include answer keys, allowing for self-correction and focused review;
Furthermore, some sets incorporate more challenging compounds featuring heterocycles like morpholine and triazole, demanding a deeper understanding of complex naming conventions. Consistent use of these sets dramatically improves proficiency.
